Associate Professor, Dept Neuroscience, Physiology & Pharmacolgy
Based at UCL
Second messenger signaling in synaptic plasticity
Changes in synaptic strength (synaptic plasticity) following synaptic transmission are thought to underlie memory formation. Synaptic plasticity is mediated by signalling enzymes on either side of the synaptic cleft that respond to changes in second messenger concentration following synaptic transmission. We are interested in resolving fundamental mechanisms that involve the action of second messengers. Questions we aim to address include:
How does cAMP-dependent protein kinase (PKA) mediate different forms of synaptic plasticity?
How can postsynaptic entry of the same second messenger (Ca2+) lead to opposite effects on synaptic strength?
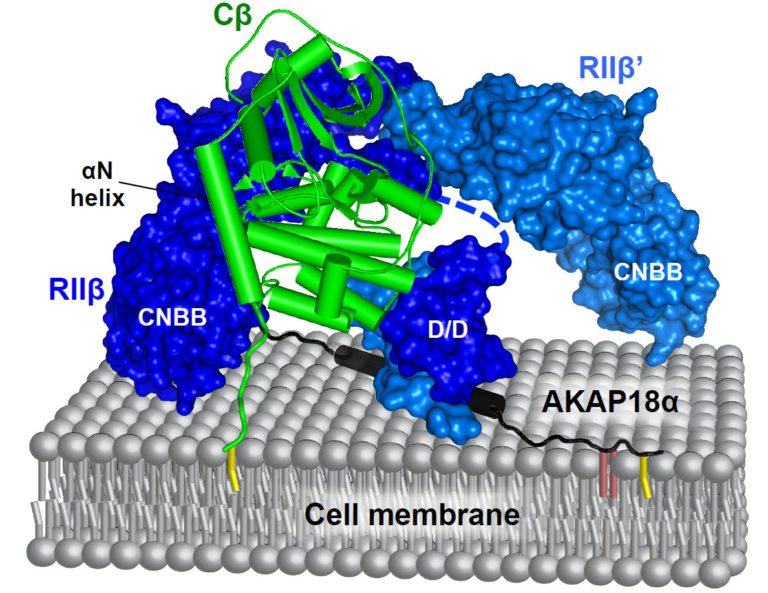
To understand how the same second messenger can have different cellular effects, and to accurately identify critical substrates of second messenger-sensitive enzymes, it is important to consider that the action of second messengers is localised within cells. Anchoring proteins position second messenger-sensitive enzymes with their substrates at subcellular locations where second messenger concentrations are periodically elevated. For example, in the case of cAMP signalling, cAMP-dependent protein kinase (PKA) is positioned in proximity to its substrates and to adenylyl cyclases by A-Kinase Anchoring Proteins (AKAPs). We are taking a multidisciplinary approach to investigate local second messenger signalling mechanisms. We study the structure of macromolecular complexes involved in local second messenger signalling using high-resolution approaches from structural biology. This strand of research is exemplified by studies of the AKAP79 complex (below). We are also developing novel technologies, using synthetic and chemical biology, for identifying the AKAPs and PKA substrates associated with different physiological processes driven by cAMP, with an emphasis on synaptic plasticity. Following this approach, we hope to illuminate both normal and pathological synaptic biology.
Selected publications
Molecular basis of interactions between CaMKII and α-actinin-2 that underlie dendritic spine enlargement. Curtis, A.J., Zhu, J., Penny, C.J., Gold, M.G. Elife (2023). https://elifesciences.org/articles/85008
AKAP79 enables calcineurin to directly suppress protein kinase A activity. Church, T.W., Tewatia, P., Hannan, S., Antunes, J., Eriksson, O., Smart, T.G., Hellgren Kotaleski, J., Gold, M.G. Elife (2021). https://elifesciences.org/articles/68164
Mechanisms for restraining cAMP-dependent protein kinase revealed by subunit quantitation and crosslinking approaches. Walker-Gray, R., Stengel, F., Gold, M.G. PNAS (2017). https://www.pnas.org/doi/10.1073/pnas.1701782114
Molecular basis of AKAP79 regulation by calmodulin. Patel, N., Stengel, F., Aebersold, R., Gold, M.G. Nature communications (2017). https://www.nature.com/articles/s41467-017-01715-w