
Senior Research Fellow, Wellcome Career Development Fellow
Based at UCL Structural and Molecular Biology Research Department
To ensure disease transmission, the malaria parasite undergoes multiple rounds of metamorphosis, as it entirely alters its cell morphology to promote uptake and establishment in the mosquito. Each round of cellular transformations is driven by an important cytoskeletal component: the cells microtubules. Microtubules provide organisation and shape while allowing cells to transport cargo, divide, move and oppose distortion. Their importance across eukaryotes and establishment as an effective drug target has resulted in their extensive study and emphasised their high structural conservation.
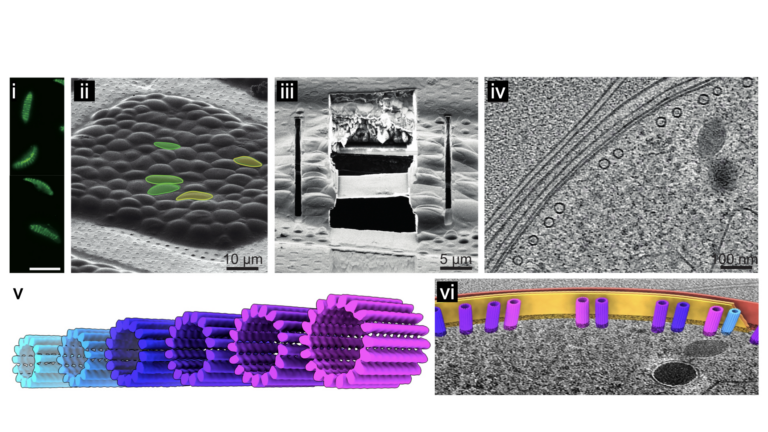
Remarkably however, we recently showed that different life cycle stages of P. falciparum have microtubules evolved to undertake specific roles, with structures that are strikingly different from the well-studied canonical microtubules in vertebrates [1]. In the transmission stage of the lifecycle, the parasite produces an incredible range of microtubule structures with variations never observed in other organisms (Fig. v). Using in situ structural biology methodologies, that span resolution and biological scales, my lab studies the structures of these non-canonical microtubules within the native parasite cell. Using a combination of cellular biology and biophysics we follow the structural work to understand how the non-canonical microtubules confer important and unique properties to the parasite.
Image below: From live cells to in situ structural parasitology. i) Parasite cells are assessed with fluorescence microscopy and then ii) vitrified on electron microscopy (EM) grids. As these are too thick to be imaged in a transmission EM, the cells are iii) micro-machined using a focussed ion beam (FIB), leaving a thin, electron-transparent lamella supported by surrounding cells. EM images at multiple angles are collected on specific regions of interest and these are computationally reconstructed into iv) 3D tomograms. Protein complexes, like these microtubules, are v) aligned and averaged to solve their structures and these can be vi) positioned back into their exact location in the 3D tomogram.
Selected publications
1. Ferreira JL, Pražák V, Vasishtan D, Siggel M, Hentzschel F, Binder AM, et al. Variable microtubule architecture in the malaria parasite. Nat Commun. 2023;14: 1216. doi:10.1038/s41467-023-36627-5
2. Ferreira JL, Frischknecht F. Parasite microtubule arrays. Curr Biol. 2023;33: R845–R850. doi:10.1016/j.cub.2023.07.001
3. Ferreira JL, Gao FZ, Rossmann FM, Nans A, Brenzinger S, Hosseini R, et al. γ-proteobacteria eject their polar flagella under nutrient depletion, retaining flagellar motor relic structures. PLOS Biol. 2019;17: e3000165. doi:10.1371/journal.pbio.3000165 depletion, retaining flagellar motor relic structures. PLOS Biol. 2019;17: e3000165. doi:10.1371/journal.pbio.3000165
