
Molecular basis of the oxidative stress response
Reactive oxygen species (ROS) are highly reactive molecules produced by cells during fundamental processes (e.g. metabolism, respiration…) or after exposure to external factors (e.g. pollution). Although ROS play an important role in many cell functions (e.g. differentiation), high levels of ROS can damage cells and alter genetic information. Therefore, cells need to control ROS levels and ensure that they remain at a healthy level. This is called maintaining the ‘redox balance’. Problems in maintaining this balance are a cause and a consequence of several human diseases, including cancer and neurodegenerative diseases. Drugs that maintain the redox balance may help to treat ROS-related diseases.
Despite their importance in human health and disease, the mechanisms underlying the redox balance remain poorly understood. We are interested in understanding how cells detect and respond to changes to the redox balance, and how these responses are deregulated, especially in cancer. We are using Structural Biology (cryo-EM and crystallography), Biophysics and Biochemistry as core techniques in the laboratory.
Selected publications
- Jenkins, T.; Gouge, J. Nrf2 in Cancer, Detoxifying Enzymes and Cell Death Programs. Antioxidants 2021, 10, 1030, doi:10.3390/antiox10071030.
- Gouge, J.; Vannini, A. New Tricks for an Old Dog: Brf2-Dependent RNA Polymerase III Transcription in Oxidative Stress and Cancer. Transcription 2017, 9, 61–66, doi:10.1080/21541264.2017.1335269.
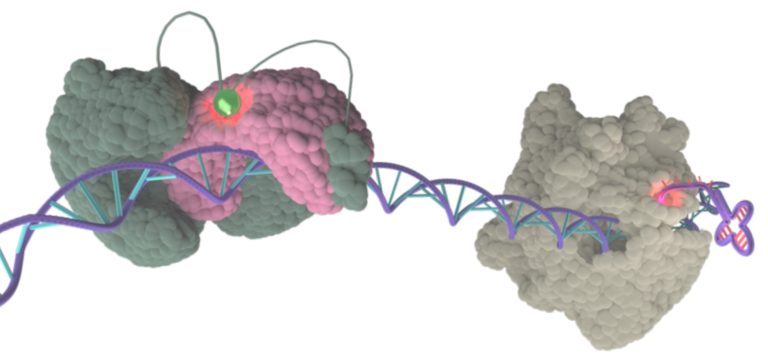
- Gouge, J.; Satia, K.; Guthertz, N.; Widya, M.; Thompson, A.J.; Cousin, P.; Dergai, O.; Hernandez, N.; Vannini, A. Redox Signaling by the RNA Polymerase III TFIIB-Related Factor Brf2. Cell 2015, 163, 1375–1387, doi:10.1016/j.cell.2015.11.005.
- Ramsay, E.P.; Abascal-Palacios, G.; Daiß, J.L.; King, H.; Gouge, J.; Pilsl, M.; Beuron, F.; Morris, E.; Gunkel, P.; Engel, C.; et al. Structure of Human RNA Polymerase III. Nat Commun 2020, 11, 6409, doi:10.1038/s41467-020-20262-5.
