
Sustainable strategies for molecular assembly using Nature's biocatalysts and the synthesis of chemical biology tools
Research activity in our group is focused on developing sustainable synthetic methods using biocatalysts in single-step reactions, multi-step cascades and performing reactions in water. Current projects include biocatalyst discovery using functional metagenomics approaches and the use of biocatalytic synthetic strategies with transaminases, transketolases, ene reductases, decarboxylases, tyrosinases, imine reductases, alcohol dehydrogenases, methyl transferases and norcoclaurine synthases. The combination of enzyme steps into biocatalytic cascades and synthetic biology pathways is a particularly efficient method for molecular assembly which we are exploring. A recent focus has also been the use of biomass waste, as a sustainable starting material to produce higher value compounds. As well as the use of enzymes for synthetic applications, we are investigating the discovery and use of enzymes for the degradation of plastics and other waste materials for molecular recycling. We are also using organic chemistry to probe and solve biological problems. Activities include: the design and synthesis of new lipid conjugates for use in nanoparticle delivery applications and as imaging reagents; a collaborative approach to understand the photoactive behaviour of GFP, and the synthesis of chemotherapeutics as anti-tuberculosis, anti-viral and anti-bacterial agents.
Research in my group has resulted in the award of prizes from the Royal Society of Chemistry and the Institute of Chemical Engineers.
Selected publications
E. Ambrose-Dempster, L. Leipold, D. Dobrijevic, M. Bawn, E. M. Carter, T. D Sheppard, G. Stojanovski, J. Jeffries, J. M. Ward, H. C. Hailes, ‘Mechanoenzymatic Reactions for the Hydrolysis of PET’, RSC Adv., 2023, 13, 9954-9962.
Y. Wang, F. Subrizi, E. M. Carter, T. D. Sheppard, J. M. Ward, H. C. Hailes, ‘Enzymatic synthesis of benzylisoquinoline alkaloids using a parallel cascade strategy and tyrosinase variants, Nature Commun., 2022, 13, 5436.
F. Subrizi, Y. Wang, B. Thair, D. Méndez-Sánchez, R. Roddan, M. Cárdenas-Fernández, J. Siegrist, M. Richter, J. N. Andexer, J. M. Ward, H C. Hailes, ‘Multienzyme one-pot cascades incorporating methyltransferases for the strategic diversification of tetrahydroisoquinoline alkaloids’, Angew. Chem. Int. Ed., 2021, 60, 18673-18679.
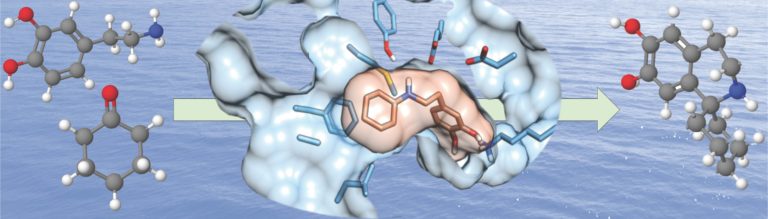
A. Maitra, D. Evangelopoulos, A. Chrzastek, L. Martin, A. Hanrath, E. Chapman, H. C. Hailes, M. Lipman, T. McHugh, S. Waddell, S. Bhakta, ‘Carprofen elicits pleiotropic mechanisms of bactericidal action with the potential to reverse antimicrobial drug resistance in tuberculosis’, J. Antimicrob. Chemother., 2020, 75, 3193-3201.
