
Pathobiology of antitrypsin deficiency and the serpinopathies
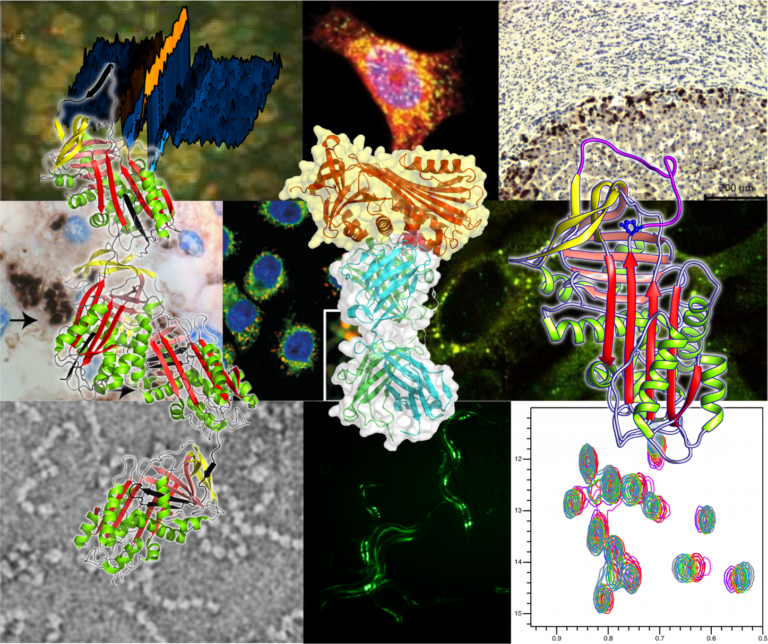
α1-antitrypsin deficiency is characterised by the misfolding and intracellular polymerisation of mutant protein within the endoplasmic reticulum of hepatocytes. The retention of mutant protein causes hepatic damage and cirrhosis whilst the lack of an important circulating protease inhibitor predisposes individuals with severe α1-antitrypsin deficiency to early onset emphysema. Our work over 25 years has described the structural basis of polymer formation and the cellular consequences. It has led to new paradigms for the liver and lung disease associated with α1-antitrypsin deficiency. The process of polymerisation is not unique to α1-antitrypsin but occurs in other members of the serine protease inhibitor (serpin) superfamily. Mutants of α1-antichymotrypsin, antithrombin, C1-inhibitor and heparin co-factor II were described by our group, and by others, that form polymers in vitro and in vivo. This is associated with emphysema, thrombosis and angiodema respectively. Perhaps most striking was our description of this process in neuroserpin to form inclusions within neurones and an autosomal dominant dementia that we named familial encephalopathy with neuroserpin inclusion bodies or FENIB. In view of the common mechanism we have grouped these diseases as a new class of disorder that we termed the serpinopathies. Our work focuses on: (i) the use of biophysical techniques, electron microscopy and NMR to understand the structural basis of pathological α1-antitrypsin polymers, (ii) the use of cell and C. elegans models to identify pathways that contribute to toxicity of the intracellular polymer and (iii) novel therapeutic strategies to block intracellular polymerisation and so treat the associated disease.
Selected publications
Hepatology (2013) 57:2049-2060
