Dissecting the molecular mechanisms of Hsp90-dependent client activation using crystallographic and cryo-EM studies
Research in my lab focuses on the molecular chaperone Hsp90. Hsp90 works in large macromolecular complexes together with cochaperones to activate and assemble a multitude of client proteins and protein complexes involved in many signalling pathways. As a consequence it is a key therapeutic target in cancer, neurodegenerative and protein folding diseases. We use structural studies, including crystallography and cryo-electron microscopy, in addition to biochemical and biophysical techniques to understand:
(1) the role of essential cochaperones in facilitating Hsp90 interactions with substrate proteins
We study the role of the cochaperone Sgt1 and its interaction with Hsp90 in facilitating the assembly of a yeast kinetochore complex, CBF3. The kinetochore is a mega-Dalton protein assembly that bridges between sister chromatids and microtubules of the spindle pole during mitosis. CBF3 associates with the centromere DNA and is therefore fundamental for kinetochore formation. To this end we are assembling early and late intermediates of CBF3 assembly for structural characterisation by cryo-EM.
(2) the importance of post-translational modification of essential cochaperones in regulating Hsp90 function
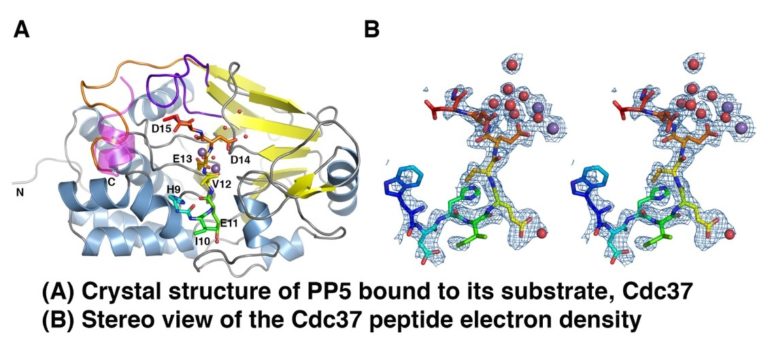
Post translational modification of Hsp90 and its cochaperones is as an important layer of regulation of Hsp90 function. We are focusing on defining the conformational changes caused by phosphorylation and dephosphorylation of the cochaperone Cdc37. Cdc37 is a kinase-specific adaptor of Hsp90, and we are particularly interested its interaction with the Hsp90-dependent phosphatase PP5 which is essential for kinase activation. In addition to conventional structural approaches, this project uses EPR spectroscopy, in collaboration with Professor Chris Kay, UCL.