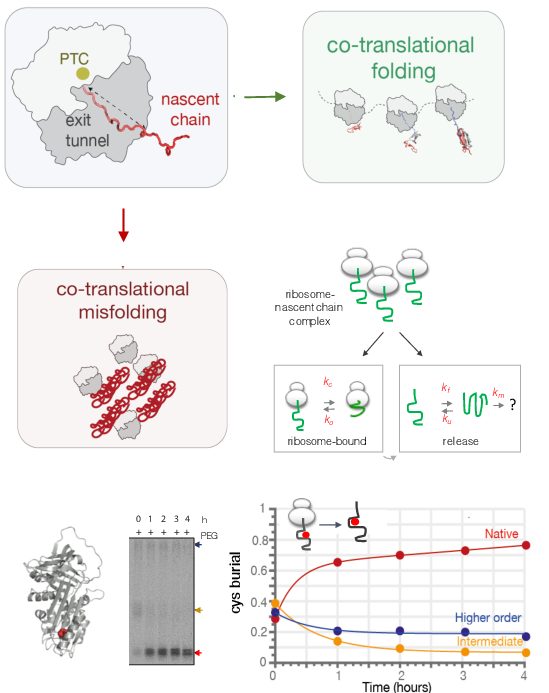
Co-translational protein misfolding and human disease
As a newly synthesised nascent polypeptide chain emerges from the ribosome during biosynthesis in the cell, it faces a choice: either folding and acquiring correct biologically-active structure co-translationally, or, folding aberrantly and forming potentially toxic structures. Although we have amassed a significant understanding of protein folding and misfolding as studied using isolated proteins, much less is understood of these processes as they occur during protein biosynthesis.
A description of the links between protein folding and misfolding processes is also important for understanding a range of human diseases, including neurodegeneration (Alzheimer's, Parkinson's, and Huntington's diseases and motor neurone disease), cancer, diabetes and respiratory diseases, the pathogenesis of which is strongly linked to protein aggregation.
Our research thus examines protein misfolding during biosynthesis at a detailed molecular level by investigating the biosynthesis, folding and misfolding of disease-associated proteins. We apply recombinant protein design strategies, including protein engineering using rational design/directed evolution approaches and site-specific gene-editing (e.g. CRISPR), together with a range of techniques including quantitative biochemistry and biophysics, as well as cell culture techniques to examine these processes as they occur in vivo. We work closely with Prof John Christodoulou's group (UCL) in a shared laboratory, using integrative structural biology approaches to generate high-resolution structures to describe co-translational misfolding processes.
Our research is supported by the AlphaOne Foundation and Motor Neurone Disease Association. We will develop contemporary models of diseases by considering the roles of protein biosynthesis and co-translational protein folding/misfolding processes