Integrative structural biology of protein folding during biosynthesis on the ribosome
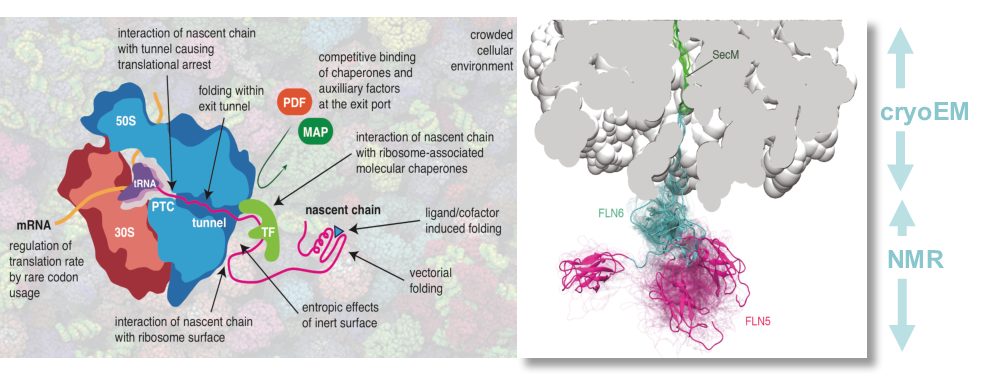
All protein synthesis occurs on the ribosome, the 2.4 MDa macromolecular machine that translates our genetic code. During protein biosynthesis, a nascent polypeptide chain exits the ribosome and has its first opportunity to fold and acquire its biologically active, tertiary structure. Our current understanding of protein folding comes from seminal studies on isolated proteins and while these have provided a backdrop, the structural and dynamic basis of this crucial folding process as it occurs co-translationally on the ribosome, within the cell remains poorly understood.
We thus use an integrative structural biology approach by combining the unique advantages offered by NMR spectroscopy, cryo-electron microscopy and molecular dynamics (MD) to provide high-resolution structures of the process of biosynthesis on the ribosome particle. We are describing the kinetic and thermodynamic basis for co-translational protein folding by examining the earliest events associated with the dynamic nascent chain as it emerges from the ribosome.
We are dissecting the molecular mechanisms of co-translational processes by combining structural techniques with protein engineering design and gene-editing strategies (e.g. CRISPR), together with analytical biochemistry and biophysics. In-cell NMR spectroscopy also enables us to study protein biosynthesis in real-time within cells. We work closely with the group of Dr. Lisa Cabrita (UCL) with whom we share a laboratory to develop novel recombinant systems, towards developing a structural description of co-translational folding and misfolding processes.
Our research, supported by a Wellcome Trust Investigator Award from 2012-2022 aims to describe all aspects of the co-translational folding landscape.